Valsartan CAS 137862-53-4 Assay 98.0~102.0% API USP Standard
Ruifu Chemical is the leading supplier of Valsartan(CAS: 137862-53-4) with high quality, can meet the USP / EP standard. Ruifu Chemical has been supplying APIs and pharmaceutical intermediates more than 15 years.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Valsartan and related intermediates, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | Valsartan |
| Synonyms | N-Valeryl-N-[2'-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl]-L-valine |
| CAS Number | 137862-53-4 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C24H29N5O3 |
| Molecular Weight | 435.52 |
| Melting Point | 116.0~117.0℃ |
| Density | 1.212±0.06 g/cm3 |
| Stability | Hygroscopic |
| Solubility | Soluble in Methanol |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Test Items | Specifications | Results |
| Appearance | White to almost white crystalline powder | White crystalline powder |
| Identification | ||
| IR | Must complies with reference standard | Complies |
| HPLC | Must complies with reference standard | Complies |
| Absorbance (420mm) | ≤0.02% (λ=420nm, C=0.05g/ml, L=1cm) | 0.012% |
| Water (by K.F) | ≤1.00% | 0.20% |
| Residue on Ignition | ≤0.10% | 0.06% |
| Heavy Metals | ≤0.001% | <0.001% |
| Related Substances | ||
| Compound A | ≤1.00% | 0.37% |
| Compound B | ≤0.20% | 0.02% |
| Compound C | ≤0.10% | Not Detectable |
| Any Other Individual Impurity | ≤0.10% | 0.02% |
| Total Impurities | ≤0.30% (Excluding R-Isomer) | 0.07% |
| Residual Solvents (GC) | ||
| Methanol | ≤3000ppm | 693ppm |
| Ethyl Acetate | ≤5000ppm | 3013ppm |
| N,N-Dimethylformamide | ≤880ppm | Not Detectable |
| Toluene | ≤890ppm | 8ppm |
| Assay / Analysis Method | 98.0~102.0% (Calculated on the Anhydrous Basis, Solvent-Free Basis) |
99.7% |
| Test Standard | USP 37 | Complies |
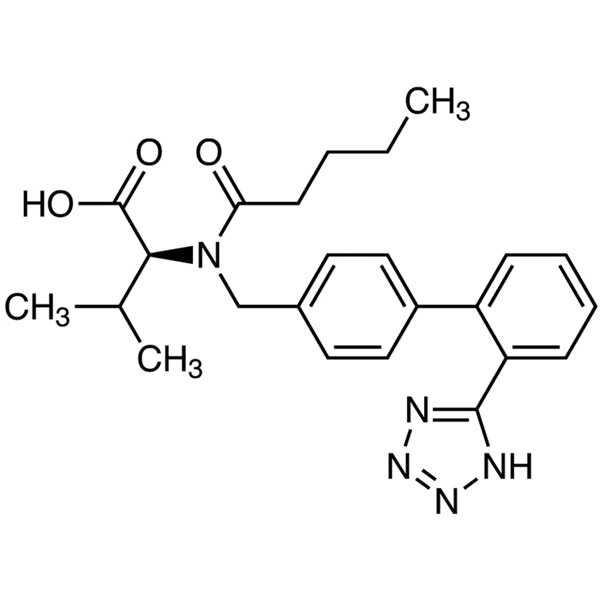
Valsartan
C24H29N5O3 435.52
l-Valine, N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-.
N-[p-(o-1H-Tetrazol-5-ylphenyl)benzyl]-N-valeryl-l-valine [137862-53-4].
Valsartan contains not less than 98.0 percent and not more than 102.0 percent of C24H29N5O3, calculated on the anhydrous basis.
Packaging and storage-Preserve in tight containers, and store at 25, excursions permitted between 15 and 30. Protect from moisture and heat.
USP Reference standards <11>-
USP Valsartan RS
USP Valsartan Related Compound A RS
(R-N-Valeryl-N-([2'-(1H-tetrazole-5-yl)biphen-4-yl]methyl)valine.
C24H29N5O3 435.52
USP Valsartan Related Compound B RS
(S-N-Butyryl-N-([2'-(1H-tetrazole-5-yl)biphen-4-yl]methyl)-valine.
C23H27N5O3 421.49
USP Valsartan Related Compound C RS
(S-N-Valeryl-N-([2'-(1H-tetrazole-5-yl)biphen-4-yl]methyl)-valine benzyl ester.
C31H35N5O3 525.64
Identification-
A: Infrared Absorption <197M>.
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Absorbance-Prepare a 1 in 20 solution in methanol, and determine the absorbance at 420 nm. The absorbance divided by the path length is not more than 0.02.
Water, Method I <921>: not more than 2.0%.
Residue on ignition <281>: not more than 0.1%.
Heavy metals, Method II <231>: 0.001%.
Related compounds-
test 1 (limit of valsartan related compound a)-
Mobile phase-Prepare a mixture of n-hexane, 2-propanol, and trifluoroacetic acid (85:15:0.1). Make adjustments if necessary (see System Suitability under Chromatography 621).
Standard solution-Dissolve an accurately weighed quantity of USP Valsartan Related Compound A RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, to obtain a solution having a known concentration of about 0.01 mg per mL.
System suitability solution-Dissolve accurately weighed quantities of USP Valsartan RS and USP Valsartan Related Compound A RS in Mobile phase to obtain a solution having known concentrations of about 0.04 mg per mL each of valsartan and valsartan related compound A.
Test solution-Transfer about 50 mg of Valsartan, accurately weighed, to a 50-mL volumetric flask, add about 40 mL of Mobile phase, and sonicate for 5 minutes. Dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L40. The flow rate is about 0.8 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between valsartan related compound A and valsartan is not less than 2.0; and the relative standard deviation, determined from the valsartan related compound A peak, for replicate injections is not more than 5%.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the percentage of valsartan related compound A in the portion of Valsartan taken by the formula:
100(CS / CU)(rU / rS)
in which CS is the concentration, in mg per mL, of USP Valsartan Related Compound A RS in the Standard solution; CU is the concentration, in mg per mL, of valsartan in the Test solution; and rU and rS are the peak responses for valsartan related compound A obtained from the Test solution and Standard solution, respectively: not more than 1.0% is found.
test 2 (limit of valsartan related compound b, valsartan related compound c, and other related compounds)-
Mobile phase-Proceed as directed in the Assay.
Standard solution-Dissolve accurately weighed quantities of USP Valsartan RS, USP Valsartan Related Compound B RS, and USP Valsartan Related Compound C RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having known concentrations of about 0.001 mg of valsartan per mL, 0.001 mg of valsartan related compound B per mL, and 0.001 mg of valsartan related compound C per mL.
Test solution-Transfer about 50 mg of Valsartan, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography <621>)-Prepare as directed in the Assay, except to use a 225-nm detector. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the resolution, R, between valsartan related compound B and valsartan is not less than 1.8; the relative standard deviation, determined from the valsartan related compound B peaks, for replicate injections is not more than 10.0%; and the relative standard deviation, determined from the valsartan peaks, for replicate injections is not more than 2.0%.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the percentage of valsartan related compound B and valsartan related compound C in the portion of Valsartan taken by the formula:
100(CS / CU)(ri / rS)
in which CS is the concentration, in mg per mL, of the appropriate USP Valsartan Related Compound RS in the Standard solution; CU is the concentration, in mg per mL, of valsartan in the Test solution; ri is the peak response for the impurity obtained from the Test solution; and rS is the peak response for the appropriate valsartan related compound obtained from the Standard solution. Calculate the percentage of each other impurity in the portion of Valsartan taken by the same formula, in which CS is the concentration, in mg per mL, of USP Valsartan RS in the Standard solution; rS is the peak response for valsartan obtained from the Standard solution; and the other terms are as defined above: not more than 0.2% of valsartan related compound B is found; not more than 0.1% of valsartan related compound C is found; not more than 0.1% of any other individual impurity, excluding valsartan related compound A, is found; and not more than 0.3% of total impurities, excluding valsartan related compound A, is found.
Assay-
Mobile phase-Prepare a filtered and degassed mixture of water, acetonitrile, and glacial acetic acid (500:500:1). Make adjustments if necessary (see System Suitability under Chromatography 621).
Standard preparation-Dissolve an accurately weighed quantity of USP Valsartan RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 0.5 mg per mL.
Assay preparation-Transfer about 50 mg of Valsartan, accurately weighed, to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 273-nm detector and a 3.0-mm × 12.5-cm column that contains 5-µm packing L1. The flow rate is about 0.4 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in mg, of C24H29N5O3 in the portion of Valsartan taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of USP Valsartan RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.

Hazard Symbols Xi - Irritant
Risk Codes
36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39 - Wear suitable gloves and eye/face protection
WGK Germany 3
RTECS YV9455000
HS Code 2933990099
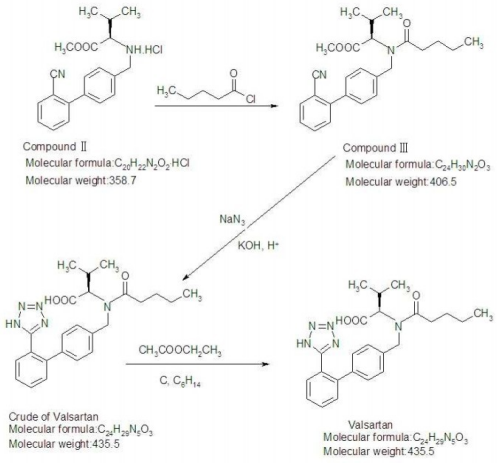
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Valsartan (CAS: 137862-53-4) with high quality. Valsartan is a nonpeptide angiotensin II AT1 receptor antagonist, Antihypertensive, has the potential for high blood pressure and heart failure research.
Valsartan’s antihypertensive effects are stronger than those of enalapril and is suitable for treating hypertension, mild to moderate primary hypertension, and especially secondary hypertension caused by renal damage. It can significantly reduce proteinuria for hypertension patients with diabetes or normal liver functions, and it can promote uric acid and urinary sodium to protect the kidney. Valsartan is also suitable for reducing the cardiovascular mortality for high risk patients (left ventricular failure or dysfunction) after experiencing a heart attack.
-
Valsartan CAS 137862-53-4 Assay 98.0~102.0% API...
-
Triphenylvalsartan CAS 7693-46-1 Purity >97.0% ...
-
L-Valine Methyl Ester Hydrochloride (H-Val-OMe·...
-
Cinchonidine CAS 485-71-2 Assay 98.5%~101.0% AP...
-
Bortezomib CAS 179324-69-7 Purity ≥99.0% (HPLC)...
-
Darunavir CAS 206361-99-1 Anti-HIV Purity ≥99.0...
-
Esmolol Hydrochloride CAS 81161-17-3 Purity ≥99...
-
Fingolimod Hydrochloride CAS 162359-56-0 Purity...
-
Lasofoxifene Tartrate CAS 190791-29-8 Chiral Pu...
-
Lurasidone Hydrochloride CAS 367514-88-3 Purity...
-
Memantine Hydrochloride Memantine HCl CAS 41100...
-
Rapamycin Sirolimus RAPA CAS 53123-88-9 Assay ≥...
-
Tacrolimus FK-506 Fujimycin CAS 104987-11-3 API...
-
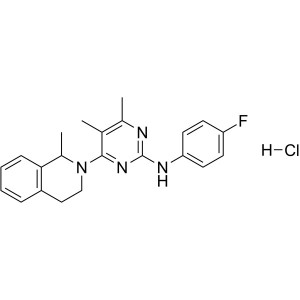
Revaprazan Hydrochloride CAS 178307-42-1 Assay ...
-
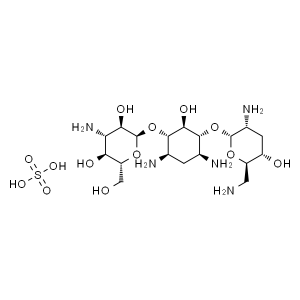
Tobramycin Sulfate CAS 49842-07-1 Potency 634μg...
-
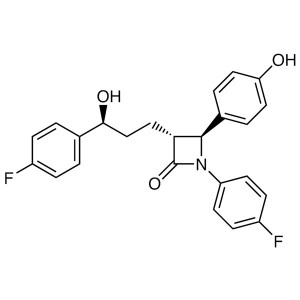
Ezetimibe CAS 163222-33-1 Purity 98.5%~102.0% (...